Applications
Hardness and Wear Resistance
Ceramic properties are determined by the choice of chemical elements, the type of bond and crystal structure. The metallic bond occurs in metals, having atomic cores surrounded by electron gas which explains their ductility and electrical conductivity, while ionic and covalent bonds occur in ceramics giving rise to strong bonding forces. This explains the high level of Young’s Modulus, hardness and melting point, the low thermal expansion as well as the brittle fracture behaviour. There is an additional mechanism in ceramics based on zirconia in particular, which has a positive influence on ceramic properties when applied correctly. A phase transformation and the development of different phases may give rise to a transformation toughening resulting in an extremely high bending strength.
There are two basic mechanisms of tribological wear - impingement wear and friction wear. With impingement wear, particles impact and erode the surface. Friction occurs when two bodies rub against each other implying punctually high local stress and pressure which results in shear stress, fracture and high local temperatures. High temperatures may even lead to fusing or chemical reactions resulting in destruction of the surface. This occurs in such devices as rotating shafts, valve seats and metal extrusion and drawing dies, i.e. components which are well suited to the use of ceramic due to its high strength.
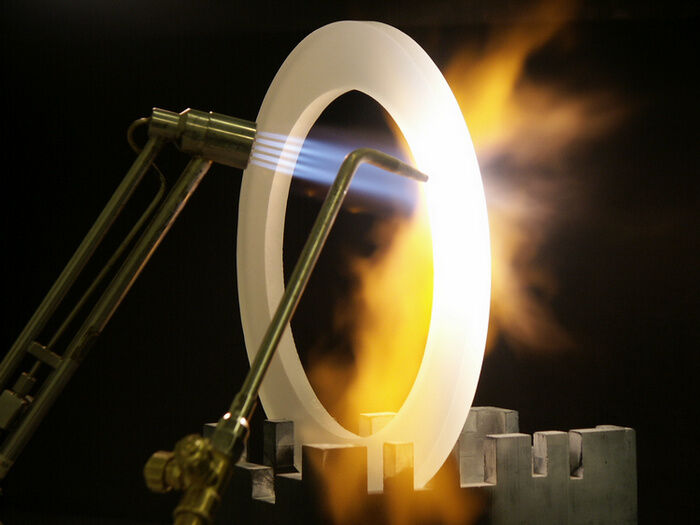
Resistance to the High Temperature
Ceramic materials play a decisive role in high- temperature applications with continuous operating temperatures ranging from 180 °C to well over 1200 °C. Due to these extreme temperatures, it is impossible to use plastic materials. Many materials are present as molten mass or even steam and when temperatures rise, metals lose their strength. This is a clear distinction between ceramic materials and metallic materials. The bending strength of silicium nitride and zirconia, for instance, can be compared to that of steel and remains almost unchanged at temperatures up to 1000 °C. However, depending on the alloy, the bending strength of steels already decreases at temperatures of 300 °C and over.
One of the oldest fields of application of ceramic is high-temperature measuring technology where it is used in thermocouple protective tubes at temperatures over 1900 °C. The good high-temperature properties are based on the high melting point of pure oxides: 2050 °C for aluminium oxide and 2600 °C for zirconium oxide.
These properties can be found in the components only if a highly pure, high-quality base material is used. Electrical insulation properties, wear resistance and stability are maintained up to 1800 °C, especially for aluminium oxide. Improved stability is attainable through higher porosity.
However, if higher requirements for thermshock resistance are imposed, silicon nitride or a porous aluminium oxide material with good resistance should be taken into consideration. Basically, large and thick-walled components are more sensitive to temperature changes than small and thin-walled shaped bodies.
Corrosion Resistance
Compared to metallic materials, oxide ceramics are considered resistant to chemical attacks particularly with regard to acid and alkaline solutions. Products made from oxide ceramics are used in chemical plants for instance, as plastic coatings and metallic materials provide insufficient resistance to corrosion in these applications.
Even if the pure basis oxide is exceptionally resistant to corrosion, under certain conditions highly pure ceramics with a purity of > 99.5% can also be subject to corrosion. The chemical composition as well as the phase distribution and structural conditions are decisive for the occurrence and degree of corrosion. Therefore, it is possible that even ceramics with nominally identical purities exhibit a completely different corrosive behaviour depending on their origin.
When examining the structures of densely sintered aluminium oxide ceramics, larger crystallites and a silicium oxide-rich phase can be seen running from inside to outside on the grain boundaries throughout the entire structure. Silicium oxide is significantly less resistant to chemical attack than aluminium oxide particularly when additional impurities from alkalis or alkaline earth oxides are present in the glass.
If a corrosive attack occurs at this glassy phase, it might not show initially because the width of these intergranular zones is mostly less than 1 μm. However, corrosion can migrate right into the ceramic body along the grain boundaries which ultimately leads to exposure of primary grains. Over a longer period, this can lead to complete destruction of the material by dissolution of the glassy phase and dissolving of the crystallites. This process is described as intergranular corrosion; the higher the proportion of silicium oxide in the structure, the more intensive the corrosion.